Craig Goergen
Craig Goergen is the Leslie A. Geddes Associate Professor and the Principal Investigator of the Cardiovascular Imaging Research Laboratory at Purdue University. His work combines advanced engineering, imaging, and biological approaches to study a variety of cardiac and vascular diseases. With funding from the US National Institutes of Health (NIH), the US National Science Foundation (NSF), the American Heart Association (AHA) and the Gates Foundation, Dr. Goergen and his team are working to improve cardiovascular disease diagnosis, treatment, and prevention, ultimately providing patients with longer and more fulfilling lives.
Dr. Goergen received a BS degree in biomedical engineering from Washington University in St. Louis, Missouri, and MS and PhD degrees in bioengineering from Stanford University, Palo Alto, California. In graduate school, Dr. Goergen worked with the Biomedical Imaging Group at Genentech in South San Francisco to study abdominal aortic aneurysm formation utilizing multiple mouse models. His postdoctoral training in molecular optical imaging at Harvard Medical School focused on cardiac disease and left ventricular remodeling. He joined the faculty at Purdue University in December of 2012 and was the recipient of the 2017 Biomedical Engineering Society (BMES) Rita Schaffer Young Investigator Award.
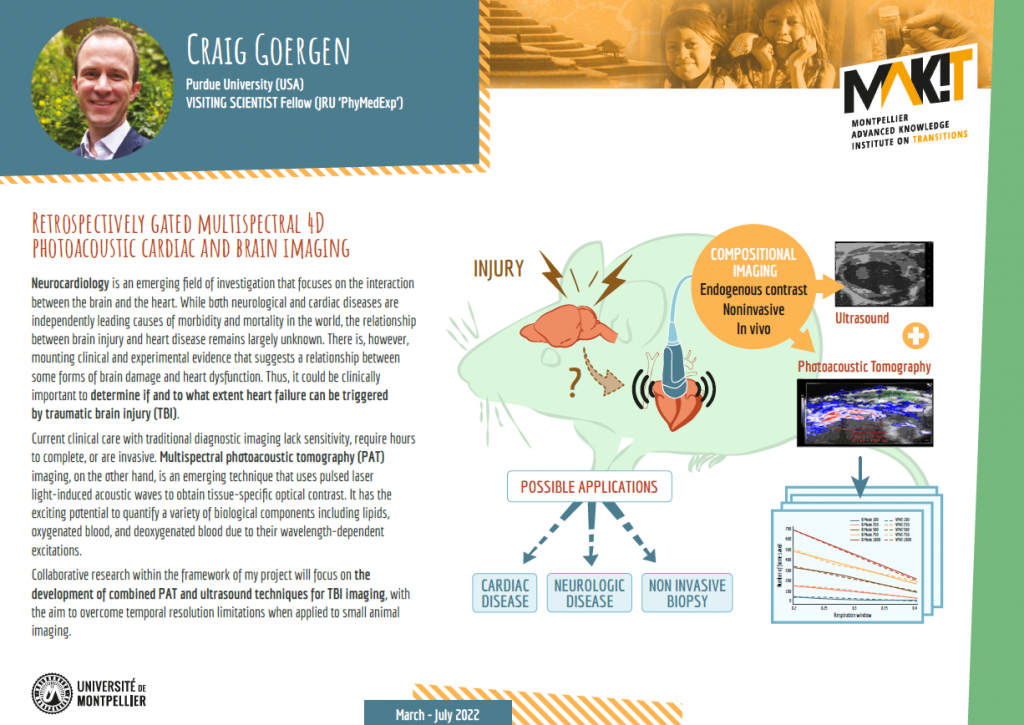
Neurocardiology is an emerging field of investigation that focuses on the interaction between the brain and the heart. While both neurological and cardiac diseases are independently leading causes of morbidity and mortality in the world, the relationship between brain injury and heart disease remains largely unknown. There is, however, mounting clinical and experimental evidence that suggests a relationship between some forms of brain damage and heart dysfunction. Thus, it could be clinically important to determine if and to what extent heart failure can be triggered by traumatic brain injury (TBI).
Current clinical care with traditional diagnostic imaging lack sensitivity, require hours to complete, or are invasive. Photoacoustic (PA) imaging, on the other hand, is an emerging technique that uses pulsed laser light-induced acoustic waves to obtain tissue-specific optical contrast. Multispectral photoacoustic tomography (PAT) imaging has the exciting potential to quantify a variety of biological components including lipids, oxygenated blood, and deoxygenated blood due to their wavelength-dependent excitations. However, its utility for small animal imaging has yet to be explored largely due to the temporal resolution limitations.
Collaborative research through the MAK’IT program will focus on the development of combined PAT and ultrasound techniques for TBI imaging to overcome these limitations.